We are excited to share that CuraWay's "Radiofrequency Ablation System" has achieved FDA 510(k) clearance!

Clinically validated in over 10,000 procedures in China, this state-of-the-art system has earned the trust of physicians across a wide range of applications. By integrating advanced technologies, it delivers exceptional precision and safety for tumor ablation and interventional therapies.
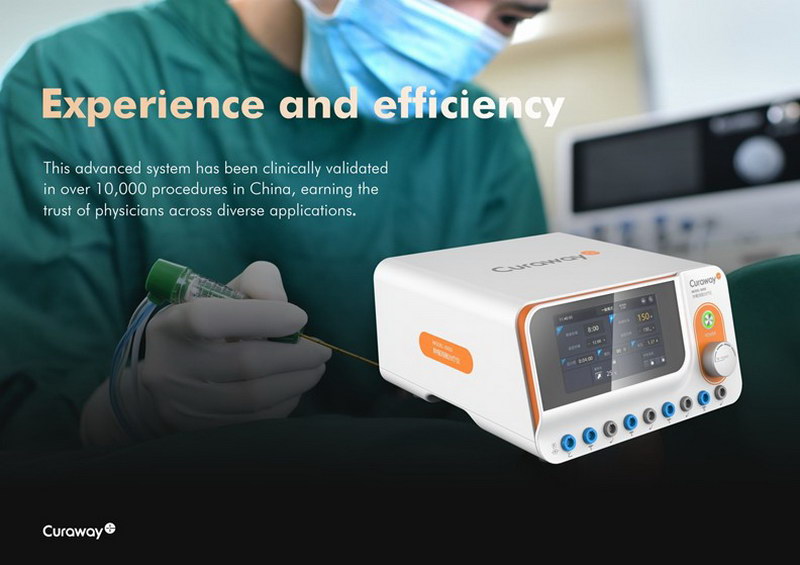
This milestone reinforces our commitment to providing high-quality, innovative medical devices on a global scale. As we look ahead to 2025, we are eager to collaborate further and bring intelligent, patient-focused solutions to meet evolving healthcare needs worldwide.
Stay tuned for more updates as we continue to expand our impact!

